Eva Kondorosi's Biography

Eva Kondorosi was born in Budapest, graduated (Biology) and received her PhD (Genetics) at the L. Eötvös University in Budapest. She was postdoc at the Max Planck Institut für Züchtungsforschung (Köln) and visiting scholar at the Sussex, Harvard and Cornell Universities. Eva Kondorosi was a founding member of the Institut des Science Végétales CNRS in Gif sur Yvette, France as one of the first research directors and group leaders and later the founding director of the BAYGEN Institute in Szeged, Hungary. Currently she directs research in the Symbiosis and Functional Genomics Unit at the Biological Research Centre of the Hungarian Academy of Sciences in Szeged.
Scientific Contribution
Her primary research field is Rhizobium-legume symbiosis but she has made significant contribution also to plant cell cycle research and developmental biology. Eva Kondorosi was amongst the first ones who identified the Rhizobium nodulation genes and contributed in many ways to the elucidation of the molecular mechanism of nodule organogenesis including cell cycle regulation. She demonstrated the importance of the endoreduplication cycles in the differentiation of plant cells and in plant development. A recent breakthrough was the discovery of plant controlled irreversible differentiation of endosymbiotic bacteria to non-cultivable polyploid nitrogen fixing bacteroids. Her group discovered 800 novel plant peptides that are responsible for terminal differentiation of the endosymbionts. Her group aims to elucidate the function of these peptides in symbiosis as well as ex planta. Strong antimicrobial activities of several peptides have opened an innovative research line for developing new antibiotics from these peptides that could effectively kill pathogenic microbes, even antibiotic resistant ones.
Scientific Recognition
For her original discoveries she received several awards including the Balzan Prize in 2018 and in 2012 the Széchenyi and the IS-MPMI awards. She has been elected to the Hungarian Academy of Sciences, National Academy of Sciences USA, French Agricultural Academy, Academia Europaea, Leopoldina, European Academy of Microbiology and EMBO. She has been Vice President of the European Research Council’s Scientific Council and the chair of the European Research Council’s Working Group on Widening European Participation. She is a member of the Academia Europaea Board of Trustees. She was a member of the United Nations Secretary General’s Scientific Advisory Board and was member of the Board of Directors of the IS-Molecular Plant-Microbe Interactions and the international jury for the OREAL-UNESCO « Women in Science » Awards.
Research Interest
In agriculture, nitrogen fertilizers provide the nitrogen source required for plant growth. Legumes have the unique capacity to establish symbiotic association with soil bacteria commonly termed rhizobia for the conversion of the inert atmospheric nitrogen gas into ammonia which can be utilized by the plants to fulfill their nitrogen needs. Nitrogen fixation takes place in the cells of de novo formed organs called nodules (Figure 1). The development of nodules requires continuous communication between the partners and is achieved through sophisticated and coordinated differentiation steps of both organisms. The efficacy of nitrogen fixation is greatest in plants where bacteria undergo an irreversible differentiation process, resulting in cell enlargement, altered morphology, increased genome size and cell membrane permeability and definitive loss of their cell division ability. The successive steps of this terminal bacteroid differentiation are plant directed and mediated by symbiotic nodule-specific cysteine-rich NCR and nodule-specific glycine-rich GRP peptides. In Medicago truncatula more than 700 NCR peptides and 25 GRP peptides are expressed at different developmental stages of the symbiotic cells and provoke sequential maturation of the endosymbiont. Many of these plant peptides act via multi-hit mechanisms by interacting with the bacterial membranes and binding to diverse bacterial targets in the cytosol. Research on the individual action of selected NCR peptides resulted in the identification of several master effectors indispensable for the endosymbionts’ differentiation. Ex planta and in vitro many cationic NCR peptides have broad ranges of antimicrobial activities effectively killing various human and plant pathogen bacteria and fungi including antibiotic resistant strains without toxicity on human cells. Selected peptides with strong antimicrobial activity on various pathogenic bacteria and fungi are currently under investigation as lead molecules for future antibiotics.
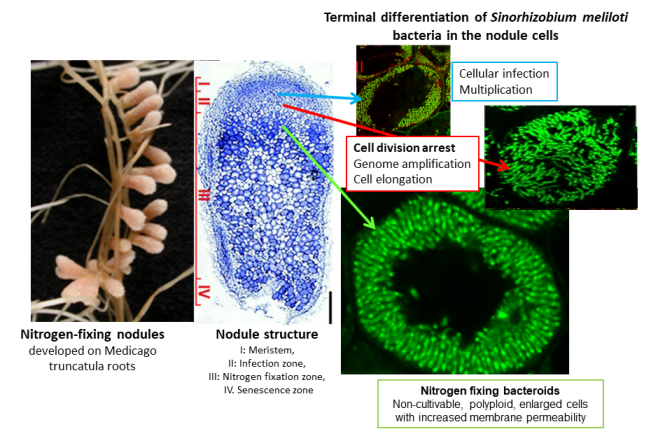
Figure 1.: Development of nitrogen-fixing root nodules in Medicago truncatula - Sinorhizobium meliloti symbiosis.