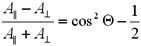|
|
|
|
|
|
 | |
Molecular motions in bacteriorhodopsin |
Principal Investigator: Pál Ormos
Vibrational spectroscopy gives detailed information about molecular structure, molecular motions. It is especially
informative to study proteins. In difference mode (where the spectrum change between functionally relevant states is
investigated) reactions of single atoms can be detected: protonation of a single group, motion of a single bond, etc.
Many important details of the action of Bacteriorhodopsin: individual proton transfer steps, protein motion crucial for
pumping could be identified.
References:
Ormos P.: Infrared spectroscopic demonstration of a conformational change in bacteriorhodopsin involved in proton pumping
Proc.Natl.Acad.Sci.USA 88 473-477 (1991)
Ormos P., Chu K. and Mourant J.: Infrared study of the L,M and N intermediates of Bacteriorhodopsin using the
photoreaction of M; Biochemistry 31 6933-6937 (1992)
Száraz S., Oesterhelt D. and Ormos P.:pH induced structural changes in Bacteriorhodopsin studied by Fourier
Transform Infrared spectroscopy, Biophys. J. 67 1706-1712 (1994)
L. Kelemen, P. Galajda, S. Száraz and P. Ormos: Chloride ion binding to bacteriorhodopsin at low pH: An infrared
spectroscopic study, Biophys. J. 76 1951-1958 (1999)
Photoselection
Polarised infrared spectroscopy gives direct structural information. If combined with photoselection (see charge motion
in 3D), and difference spectroscopy based on kinetic experiments is applied, orientation of very small units can be
determined. Comparing structural information with available X-ray structural data characteristic IR bands could be
identified. We even determined, which of the carbon atoms on a carboxylic residue protonates during a protonation
reaction – not possible to see by X-ray crystallography.
The geometry of the system:
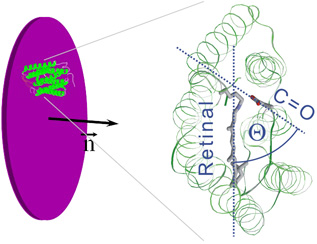
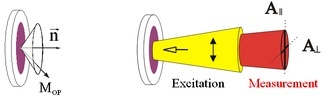
Dry purple membranes: cylindrical symmetry, retinals on a cone (left).
Start the photocycle with polarized visible light, measure with polarized IR light in two directions (right).
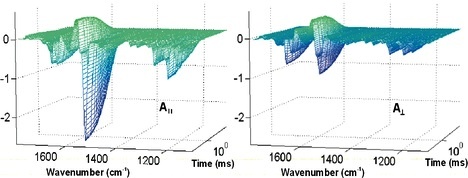
Result: difference-spectrum in time resolution.
Interpretation
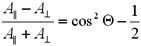
Teta is the angle between the retinal and the vibration dipole.
Reference:
L. Kelemen and P. Ormos, Conformational changes during the bacteriorhodopsin photocycle studied by time resolved FTIR
spectroscopy, Biophys.J. 81: 3577-3589 (2001)
| |