|
|
|
|
|
|
 | |
Absorption kinetics on proteins of bioenergetics |
Principal Investigator: László Zimányi
Electron transfer proteins consist of redox cofactors capable of changing their oxidation state (serving as temporary
electron traps) and a protein matrix which facilitates intra- and interprotein electron transfer. Cytochrome c (e.g.
horse heart or yeast iso-1) is one of the simplest redox proteins with a single covalently linked c-type heme cofactor.
The photoactive redox label TUPS (8-Thiouredopyrene-1,3,6-trisulfonate) has been covalently attached to various surface
lysine and genetically engineered cysteine side chains in order to initiate electron transfer reactions within this
protein.
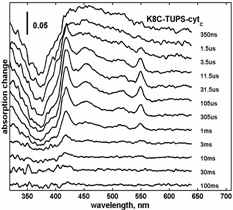
Electron transfer rate is measured by detecting time resolved absorption changes (multichannel and single wavelength)
following the laser flash excitation of TUPS. The TUPS*T triplet excited state serves as electron donor to the heme,
and the ensuing TUPS+ radical as electron acceptor.
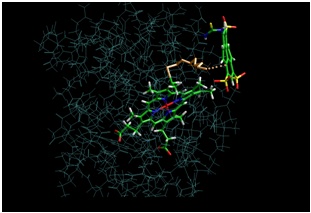
The rate of the electron transfer does not correlate with the distance between TUPS and the heme as measured along the
covalent link. MD calculations suggest multiple conformations of the TUPS-cytochrome molecule with optimal pathways
(calculated with HARLEM, www.kurnikov.org) starting as a through space jump between TUPS and the protein surface.
References:
A. B. Kotlyar, N. Borovok, P. Khoroshyy, K. Tenger, and L. Zimányi, Redox photochemistry of
thiouredopyrenetrisulfonate, Photochem. Photobiol. 79(6): 489-493 (2004)
K. Tenger, P. Khoroshyy, B. Leitgeb, G. Rákhely, N. Borovok, A. Kotlyar, D. A. Dolgikh, and L. Zimányi,
Complex kinetics of the electron transfer between the photoactive redox label TUPS and the heme of cytochrome c,
J. Chem. Inf. Mod. 45(6): 1520-1526 (2005)
| |


